The hydrogen is attached directly to a highly electronegative atoms, causing the hydrogen to acquire a highly positive charge. Each of the highly electronegative atoms attains a high negative charge and has at least one 'active' lone pair.
- Hydrogen gas is supplied to the anode of the fuel cell. The anode is coated with platinum, which acts as a catalyst to break down the hydrogen into protons and electrons. If a circuit is connected between the anode and cathode then the electrons can travel through the circuit and provide power to any load that is connected as part of the circuit.
- Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.
- Hydrogen is the first element in the periodic table. It is the simplest possible atom composed of one proton in the nucleus which is orbited by a single electron. Hydrogen is the lightest of the elements and is the most abundant element in the universe.
- In the H2 molecule, 2 hydrogen atoms share 2 electrons. The 1st shell is full, so the hydrogen molecule has a stable electron configuration. This is similar to the elements is the noble gas group, which is the last column of the periodic table.
Learning Outcomes
- Describe the respiratory chain (electron transport chain) and its role in cellular respiration
You have just read about two pathways in cellular respiration—glycolysis and the citric acid cycle—that generate ATP. However, most of the ATP generated during the aerobic catabolism of glucose is not generated directly from these pathways. Rather, it is derived from a process that begins with moving electrons through a series of electron transporters that undergo redox reactions: the electron transport chain. This causes hydrogen ions to accumulate within the matrix space. Therefore, a concentration gradient forms in which hydrogen ions diffuse out of the matrix space by passing through ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.

Figure 1. The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
The electron transport chain (Figure 1) is the last component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants; in animals, it enters the body through the respiratory system. Electron transport is a series of redox reactions that resemble a relay race or bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain where the electrons reduce molecular oxygen, producing water. There are four complexes composed of proteins, labeled I through IV in Figure 1, and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. Note, however, that the electron transport chain of prokaryotes may not require oxygen as some live in anaerobic conditions. The common feature of all electron transport chains is the presence of a proton pump to create a proton gradient across a membrane.
Complex I
To start, two electrons are carried to the first complex aboard NADH. This complex, labeled I, is composed of flavin mononucleotide (FMN) and an iron-sulfur (Fe-S)-containing protein. FMN, which is derived from vitamin B2, also called riboflavin, is one of several prosthetic groups or co-factors in the electron transport chain. A prosthetic group is a non-protein molecule required for the activity of a protein. Prosthetic groups are organic or inorganic, non-peptide molecules bound to a protein that facilitate its function; prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase and is a very large protein, containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space, and it is in this way that the hydrogen ion gradient is established and maintained between the two compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH2, which does not pass through complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced, (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from complex I and the electrons derived from FADH2 from complex II, including succinate dehydrogenase. This enzyme and FADH2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH2 electrons. The number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe++ (reduced) and Fe+++ (oxidized). The heme molecules in the cytochromes have slightly different characteristics due to the effects of the different proteins binding them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time).
Complex IV
The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H2O). The removal of the hydrogen ions from the system contributes to the ion gradient used in the process of chemiosmosis.
Chemiosmosis
In chemiosmosis, the free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions’ positive charge and their aggregation on one side of the membrane.
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Recall that many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through an integral membrane protein called ATP synthase (Figure 2). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion gradient.
Practice Question
Figure 2. ATP synthase is a complex, molecular machine that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi). (Credit: modification of work by Klaus Hoffmeier)
Dinitrophenol (DNP) is an uncoupler that makes the inner mitochondrial membrane leaky to protons. It was used until 1938 as a weight-loss drug. What effect would you expect DNP to have on the change in pH across the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug?
Show AnswerChemiosmosis (Figure 3) is used to generate 90 percent of the ATP made during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall result of these reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium, and water is formed.
Practice Question
Figure 3. In oxidative phosphorylation, the pH gradient formed by the electron transport chain is used by ATP synthase to form ATP.
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the pH of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis?
Show AnswerATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria. (The NADH generated from glycolysis cannot easily enter mitochondria.) Thus, electrons are picked up on the inside of mitochondria by either NAD+ or FAD+. As you have learned earlier, these FAD+ molecules can transport fewer ions; consequently, fewer ATP molecules are generated when FAD+ acts as a carrier. NAD+ is used as the electron transporter in the liver and FAD+ acts in the brain.
Another factor that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For example, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Moreover, the five-carbon sugars that form nucleic acids are made from intermediates in glycolysis. Certain nonessential amino acids can be made from intermediates of both glycolysis and the citric acid cycle. Lipids, such as cholesterol and triglycerides, are also made from intermediates in these pathways, and both amino acids and triglycerides are broken down for energy through these pathways. Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy contained in glucose.
In Summary: Electron Transport Chain
The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism. The electron transport chain is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. The electrons are passed through a series of redox reactions, with a small amount of free energy used at three points to transport hydrogen ions across a membrane. This process contributes to the gradient used in chemiosmosis. The electrons passing through the electron transport chain gradually lose energy, High-energy electrons donated to the chain by either NADH or FADH2 complete the chain, as low-energy electrons reduce oxygen molecules and form water. The level of free energy of the electrons drops from about 60 kcal/mol in NADH or 45 kcal/mol in FADH2 to about 0 kcal/mol in water. The end products of the electron transport chain are water and ATP. A number of intermediate compounds of the citric acid cycle can be diverted into the anabolism of other biochemical molecules, such as nonessential amino acids, sugars, and lipids. These same molecules can serve as energy sources for the glucose pathways.
Contribute!

The key difference between hydrogen atom and hydrogen ion is that the hydrogen atom is neutral whereas the hydrogen ion carries a charge.
Hydrogen is the first and the smallest element in the periodic table and is denoted as H. It is categorized under group 1 and period 1 in the periodic table because of its electron configuration: 1s1. Hydrogen can take up an electron to form a negatively charged ion, or can easily donate the electron to produce a positively charged proton. If not, it can share the electron to make covalent bonds.
The elements in the periodic table are not stable except the noble gases. Therefore, elements try to react with other elements in order to gain the noble gas electron configuration and achieve stability. Similarly, hydrogen also has to obtain an electron to achieve the electron configuration of the noble gas, Helium. When achieving this electron configuration, it forms the hydrogen ion.
CONTENTS
1. Overview and Key Difference
2. What is a Hydrogen Atom
3. What is a Hydrogen Ion
4. Side by Side Comparison – Hydrogen Atom vs Hydrogen Ion in Tabular Form
5. Summary
What is a Hydrogen Atom?
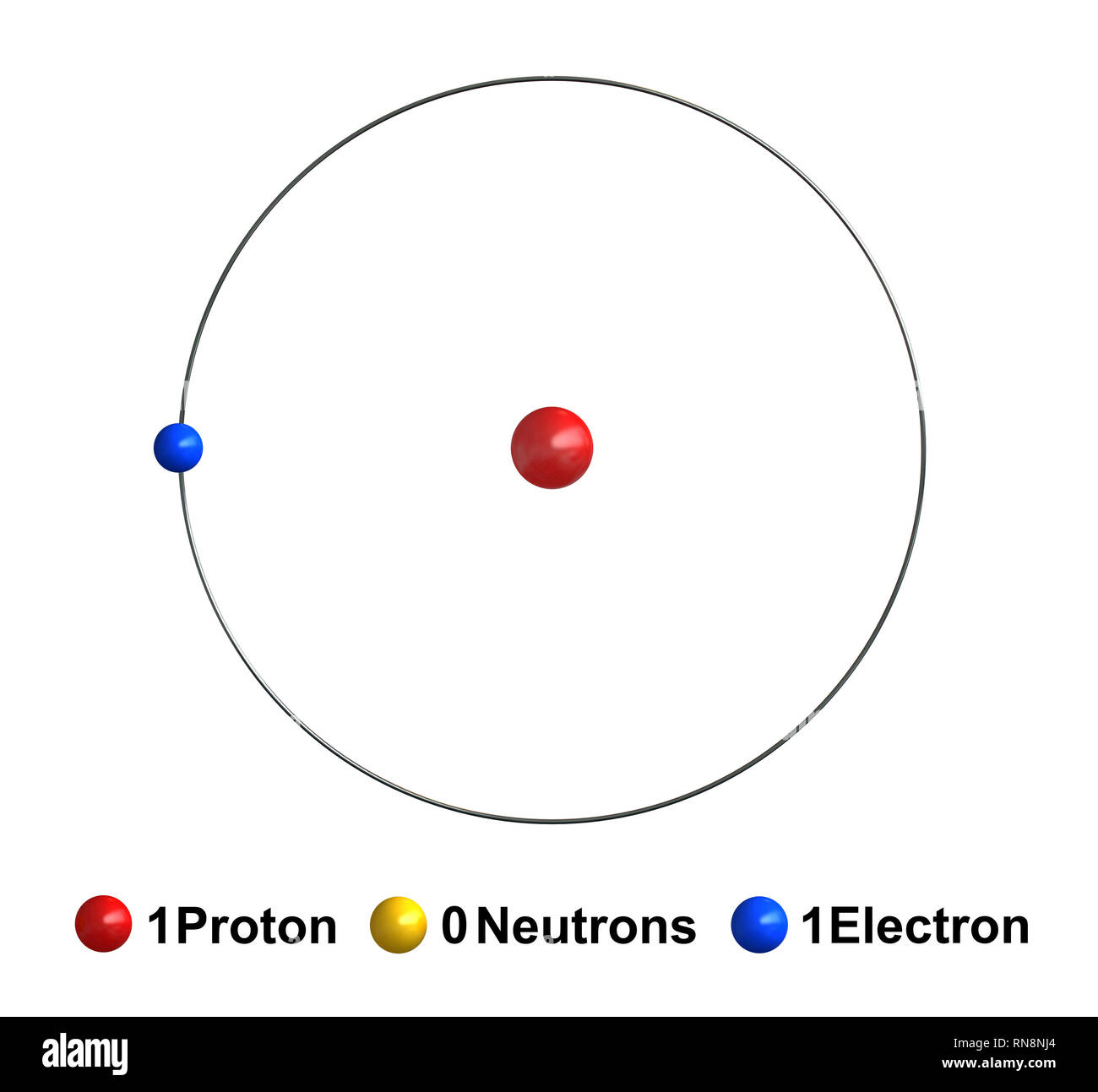
Hydrogen atom is the first element in the periodic table. A hydrogen atom has one electron and one proton. Therefore, its electron configuration is 1s1. Moreover, it only has one electron in the s-suborbital though this orbital can accommodate two electrons. Thus, hydrogen atom is unstable and very reactive in order to obtain a stable electron configuration.
Since the number of protons and electrons in a hydrogen atom are similar, this atom does not carry a net charge. Therefore, we say it is neutral. However, there are three isotopes of hydrogen: protium-1H (no neutrons), deuterium-2H (one neutron), and tritium- 3H (two neutrons). These isotopes have varying numbers of neutrons in the atomic nucleus.
What is a Hydrogen Ion?
Hydrogen ion is the form of hydrogen element that carries a charge. The charge of this ion can be either positive or negative, depending on the way that it forms. It may form from either the removal of one electron from atomic hydrogen or from electron gaining. Therefore, hydrogen ion has either a +1 or -1 charge (monovalent). We can denote the positively charged hydrogen ion as H+ (cation) and the negative ion as H- (anion).
Hydrogen Electrons Number
Figure 2: Formation of Ions from Hydrogen Atom
The cation of protium is specifically known as protons, and they are the type of hydrogen atoms we mainly consider in chemical reactions since the natural abundance of protium is very high compared to other isotopes. Further, this exists in aqueous solutions as hydronium ions (H3O+).
Hydrogen ions are responsible for acidity, and the concentration of hydrogen ions is taken to calculate pH values. When hydrogen atoms react with other nonmetals, hydrogen ions are formed, and these are released to the aqueous medium completely or partially when the molecule is dissolved. Although the formation of hydrogen anion is rare, it forms when hydrogen reacts with metals such as group 1 metals.
Hydrogen Electrons Amount
What is the Difference Between Hydrogen Atom and Hydrogen Ion?
Hydrogen is the smallest chemical element. It has one proton and one electron, making it neutral. However, the ions of hydrogen atom are charged species. Thus, the key difference between hydrogen atom and hydrogen ion is that the hydrogen atom is neutral whereas hydrogen ion carries a charge. Another significant difference between hydrogen atom and hydrogen ion is that hydrogen atom has one electron while cation of hydrogen has no electrons and anion of hydrogen has two electrons.
Moreover, hydrogen atom is highly reactive in order to obtain a stable electron configuration. But, hydrogen ions are less/not reactive since they have already gained the stable state. The charge of the cation is +1, and the charge of anions is -1. So, we can consider this also as a difference between hydrogen atom and hydrogen ion.
Summary – Hydrogen Atom vs Hydrogen Ion
Hydrogen is the first element in the periodic table of elements. Therefore, it is the smallest atom. It can form either positively charged or negatively charged ions. The key difference between hydrogen atom and hydrogen ion is that the hydrogen atom is neutral whereas hydrogen ion carries a charge.
Reference:
1. JOHN C. MORRISON, in Modern Physics, 2010
2. “8.1: The Hydrogen Atom.” Physics LibreTexts, Libretexts, 2 May 2019, Available here.
Image Courtesy:
1. “2750576” (CC0) via Pixabay
2. “Ions” By Jkwchui – Own work (CC BY-SA 3.0) via Commons Wikimedia
Hydrogen Electron Configurations
Related posts:
